Our research projects
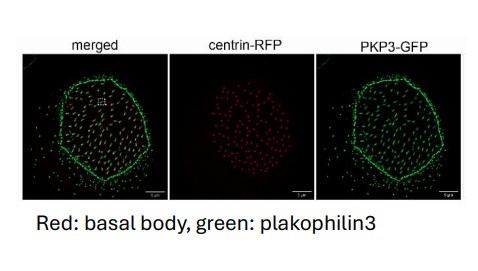
The role of cytoskeletal cross-linkers in vertebrate multiciliated cells. (Project funded under RESTART2016-2020, EXCELLENCE HUBS, excellence/0421/0305).
Project Overview
Multiciliated cells generate unidirectional fluid flow to move the cerebrospinal fluid in the brain, mucus in the airway, egg in the oviduct and sperm in the efferent ducts. Defects in the development or function of these cells lead to diseases such as primary ciliary dyskinesia. The fluid flow is generated by hundreds of motile cilia, microtubule based organelles, that project from the cell surface. Cilia must be properly spaced and oriented on the apical surface in order to beat co-ordinately in the same direction. The mechanisms that control the organization of cilia in multicliated cells are not well understood. In this project we reveal a novel role for the cell adhesion protein plakophilin 3 in the function of multiciliated cells. We specifically found that plakophilin 3 localizes at the base of cilia and it is important for positioning the basal body, the MTOC for cilia, at the apical surface of the cell.
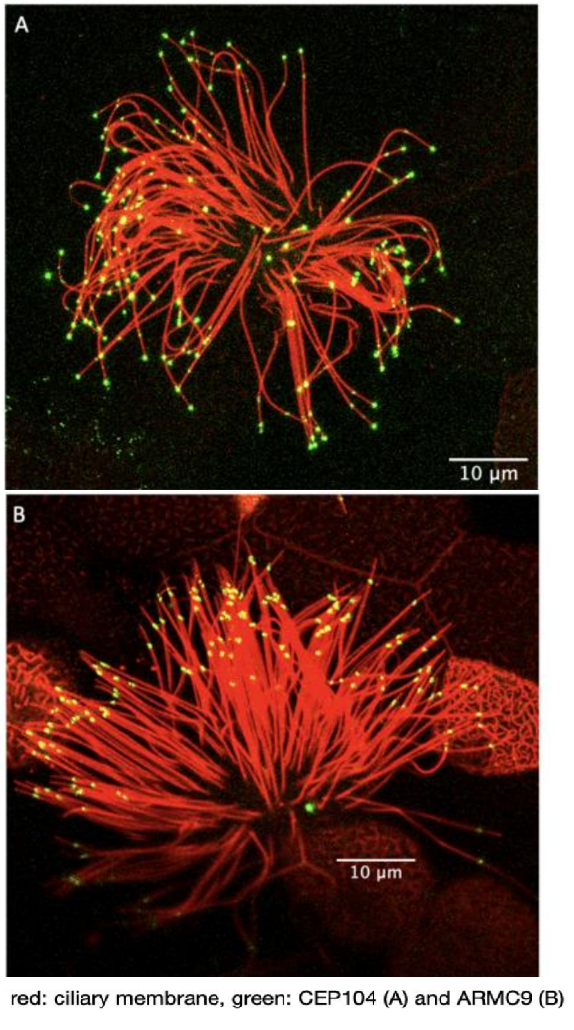
Marie Skłodowska-Curie Individual Fellowship: The role of ciliary tip organization in hedgehog signaling (proposal number 893418)
Project overview
This project examined the role of ciliary microtubule organization in hedgehog signaling. Cilia are essential motile and sensory organelles, with a sophisticated microtubule-based core structure (called the axoneme). The axoneme is made of compound outer microtubules that transition from a triplet organization at the base (A-, B- and C-tubules), to doublet organization in the middle segment (A- and B-tubules) and singlet organization (A-tubules) near the distal end of the cilium. These structural changes define the cilium geometry and correlate with the compartmentalization of various processes. Three proteins are so far known to control the length of ciliary microtubules in the protist Tetrahymena (CEP104, ARMC9 and CHE-12). Defects in these proteins affect hedgehog signaling in cell culture and in humans they cause a subset of cases of Joubert Syndrome, a neurodevelopmental ciliopathy. This raises the possibility that the ciliary geometry is important for hedgehog signaling and embryonic development. To test this, we examined the ciliary microtubule organization in the absence and presence of hedgehog signaling and the role of CEP104 and ARMC9 in hedgehog signaling and Xenopus embryonic development.
Our first aim was to explore a potential role of the ciliary microtubule organization in Hedgehog signaling. To do this we generated strong evidence of the specificity of ARMC9 for the ends of B- tubules in Xenopus. We examined the termination points of B-tubules, in the primary cilia of the neural tube in Xenopus embryos, in the absence and presence of a hedgehog signaling chemical inhibitor. When Hedgehog signaling was blocked the spread of ARMC9 signal at the ciliary tip was decreased and the overall protein levels were increased. The change in ARMC9 localization suggests that B-tubules terminate closer to each other in the absence of hedgehog signaling confirming that there is a link between the ciliary tip organization and hedgehog signaling.
Our second aim was to determine the role of CEP104 and ARMC9 in hedgehog signaling and embryonic development. We first confirmed that the localization and function of CEP104 and ARMC9 are conserved in the cilia of Xenopus. In the primary cilia of the neural tube both proteins localize to the tip of the cilium suggesting the presence of a very short ciliary tip that cannot be resolved using fluorescence microscopy. Downregulation of CEP104 and ARMC9 led to the development of embryos with microphthalmia, fewer melanocytes and neuronal abnormalities suggesting defects in hedgehog signaling. In addition, embryos showed defects in the pronephros that suggested defects in cilia generated fluid flow. Thus, we examined the effect of CEP104 and ARMC9 on cilia generated fluid flow using the embryonic epidermis as a model. Downregulation of CEP104 caused a delay in neural tube closure which is likely caused by defects in cytoplasmic microtubules. Overall, we show that 1) there is a link between the ciliary tip organization in neural tube cilia and hedgehog signaling, 2) we show that CEP104 and ARMC9 localization and function are conserved in vertebrates, 3) CEP104 and ARMC9 play a role in Hedgehog signaling and neurodevelopment in vivo and 4) uncovered novel roles of CEP104 on cytoplasmic microtubules.
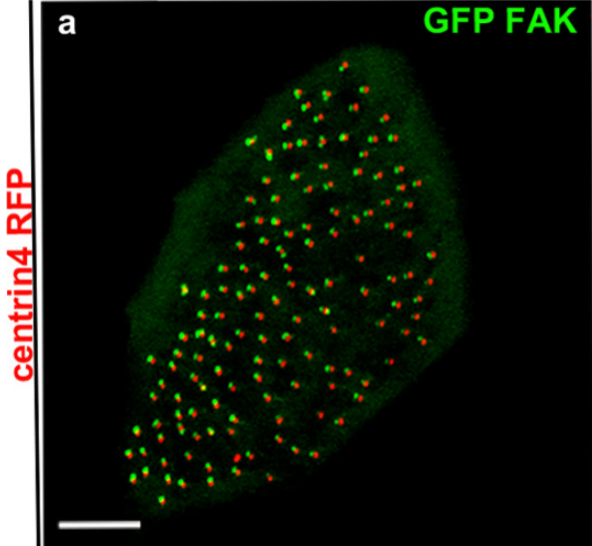
Focal adhesion proteins localization at cilia
Cilia are microtubule-based organelles involved in cell signalling, locomotion and generation of extracellular fluid flow. Xenopus laevis is a powerful model for the study of motile cilia because of the large number of multiciliated cells that decorate its epithelium. Previous work from our lab led to the discovery of ciliary adhesions (CA) which include the focal-adhesion proteins FAK, Paxillin and Vinculin. CAs are multiprotein complexes which associate with the basal bodies of cilia and play a critical role in the interactions between basal bodies and the actin cytoskeleton during multicilated cell differentiation. Current work focuses on the precise localization of the CA complex in relation to ciliary accessory structures and identification of new CA protein members. Further work includes the development of in vitro assays to study mucociliary clearance and assess the effect of candidate drugs in order to treat cilia-associated diseases (ciliopathies).
Marie Skłodowska-Curie Individual Fellowship: Addressing the impact of surface ectoderm and somitic mesoderm development on neural tube morphogenesis (proposal number 101038073)
Neural tube closure is a fundamental process during vertebrate embryogenesis, which leads to the formation of the central nervous system. Defective neural tube closure leads to neural tube defects, which are one of the most common human birth defects. In our lab we aim to delineate the contribution of distinct morphogenetic processes during neural tube closure. To achieve this, we employ an interdisciplinary research programme which includes live imaging of Xenopus Laevis embryos, 4D cell tracking, optogenetic manipulation of cell contractility and loss of function approaches.
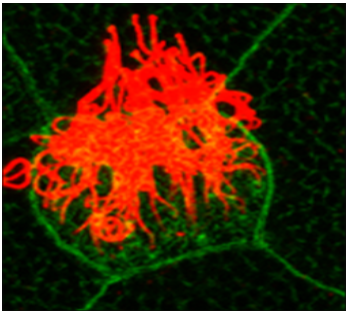
Primary cilia and hedgehog signaling
Cilia are microtubule based organelles that project from the cell surface. There are two types of cilia, motile and primary cilia. Typically, a single primary cilium projects from the surface of almost all cells in the human body. Primary cilia are mainly involved in cilia-based signaling such as hedgehog signaling, which is essential for vertebrate development. We are interested in understanding how primary cilia support hedgehog signaling.
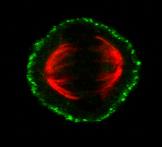
Mitosis and the Cortical Mechanosensory Complex (CMC)
Mitotic spindle orientation (SO) is a strictly regulated process which is important both during embryonic development and in the adult organism. The formation of the bipolar spindle ensures equal segregation of chromosomes to the daughter cells, whereas the orientation of the spindle determines cell fate and cell daughter positioning. Therefore, it is involved in proper tissue homeostasis, and generation and maintenance of tissue architecture. Work from our group has uncovered the presence of a mechanosensory complex involved in spindle orientation. Our group has shown that ligand independent force dependent integrin activation takes place at the lateral cortex of the mitotic cell in a polarized manner at the areas that receive the strongest force, and downstream promotes the recruitment of focal adhesion proteins at the cortex such as FAK, p130Cas and Src. Our goal is to determine the members of the CMC and understand how it is involved in spindle orientation.
