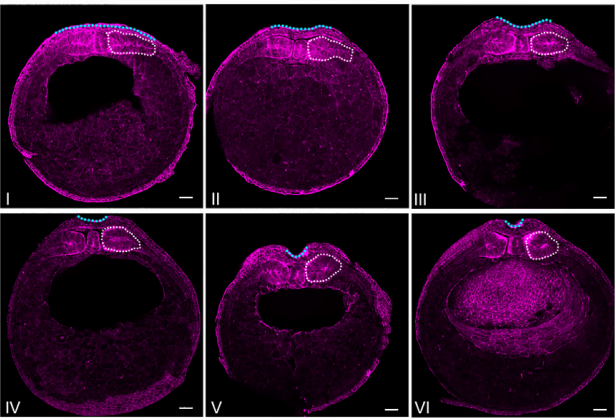
Somitic mesoderm morphogenesis is necessary for neural tube closure during Xenopus development
2023
Neophytos Christodoulou and Paris A. Skourides
Frontiers in Cell and Developmental Biology
Neural tube closure is a fundamental process during vertebrate embryogenesis, which leads to the formation of the central nervous system. Defective neural tube closure leads to neural tube defects which are some of the most common human birth defects. While the intrinsic morphogenetic events shaping the neuroepithelium have been studied extensively, how tissues mechanically coupled with the neural plate influence neural tube closure remains poorly understood. Here, using Xenopus laevis embryos, live imaging in combination with loss of function experiments and morphometric analysis of fixed samples we explore the reciprocal mechanical communication between the neural plate and the somitic mesoderm and its impact on tissue morphogenesis. We show that although somitic mesoderm convergent extension occurs independently from neural plate morphogenesis neural tube closure depends on somitic mesoderm morphogenesis. Specifically, impaired somitic mesoderm remodelling results in defective apical constriction within the neuroepithelium and failure of neural tube closure. Last, our data reveal that mild abnormalities in somitic mesoderm and neural plate morphogenesis have a synergistic effect during neurulation, leading to severe neural tube closure defects. Overall, our data reveal that defective morphogenesis of tissues mechanically coupled with the neural plate can not only drastically exacerbate mild neural tube defects that may arise from abnormalities within the neural tissue but can also elicit neural tube defects even when the neural plate is itself free of inherent defects.
A new mechanochemical model for apical constriction: Coupling calcium signalling and viscoelasticity
2022
Katerina Kaouri, Neophytos Christodoulou, Abhishek Chakraborty, Paul E Méndez, Paris Skourides, Ricardo Ruiz-Baier
Frontiers in Systems Biology
Embryonic epithelial cells exhibit strong coupling of mechanical responses to chemical signals and most notably to calcium. Recent experiments have shown that the disruption of calcium signals during neurulation strongly correlates with the appearance of neural tube defects. We, thus, develop a multi-dimensional mechanochemical model and use it to reproduce important experimental findings that describe anterior neural plate morphogenetic behaviour during neural tube closure. The governing equations consist of an advection-diffusion-reaction system for calcium concentration which is coupled to a force balance equation for the tissue. The tissue is modelled as a linear viscoelastic material that includes a calcium-dependent contraction stress. We implement a random distribution of calcium sparks that is compatible with experimental findings. A finite element method is employed to generate numerical solutions of the model for an appropriately chosen range of parameter values. We analyse the behaviour of the model as three parameters vary: the level of IP3 concentration, the strength of the stretch-sensitive activation and the maximum magnitude of the calcium-dependent contraction stress. Importantly, the simulations reproduce important experimental features, such as the spatio-temporal correlation between calcium transients and tissue deformation, the monotonic reduction of the apical surface area and the constant constriction rate, as time progresses. The model could also be employed to gain insights into other biological processes where the coupling of calcium signalling and mechanics is important, such as carcinogenesis and wound healing.
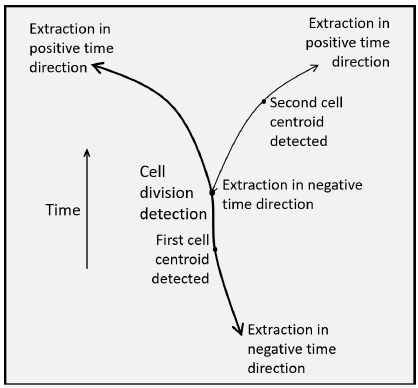
Spatiotemporal Identification of Cell Divisions Using Symmetry Properties in Time-Lapse Phase Contrast Microscopy
30/August/2022
Stathis Hadjidemetriou, Rania Hadjisavva, Andri Christodoulou, Ismini Papageorgiou, Ioanna Panayiotou, Paris Skourides
Symmetry
A variety of biological and pharmaceutical studies, such as for anti-cancer drugs, require the quantification of cell responses over long periods of time. This is performed with time-lapse video microscopy that gives a long sequence of frames. For this purpose, phase contrast imaging is commonly used since it is minimally invasive. The cell responses of interest in this study are the mitotic cell divisions. Their manual measurements are tedious, subjective, and restrictive. This study introduces an automated method for these measurements. The method starts with preprocessing for restoration and reconstruction of the phase contrast time-lapse sequences. The data are first restored from intensity non-uniformities. Subsequently, the circular symmetry of the contour of the mitotic cells in phase contrast images is used by applying a Circle Hough Transform (CHT) to reconstruct the entire cells. The CHT is also enhanced with the ability to “vote” exclusively towards the center of curvature. The CHT image sequence is then registered for misplacements between successive frames. The sequence is subsequently processed to detect cell centroids in individual frames and use them as starting points to form spatiotemporal trajectories of cells along the positive as well as along the negative time directions, that is, anti-causally. The connectivities of different trajectories enhanced by the symmetry of the trajectories of the daughter cells provide as topological by-products the events of cell divisions together with the corresponding entries into mitoses as well as exits from cytokineses. The experiments use several experimental video sequences from three different cell lines with many cells undergoing mitoses and divisions. The quantitative validations of the results of the processing demonstrate the high performance and efficiency of the method.
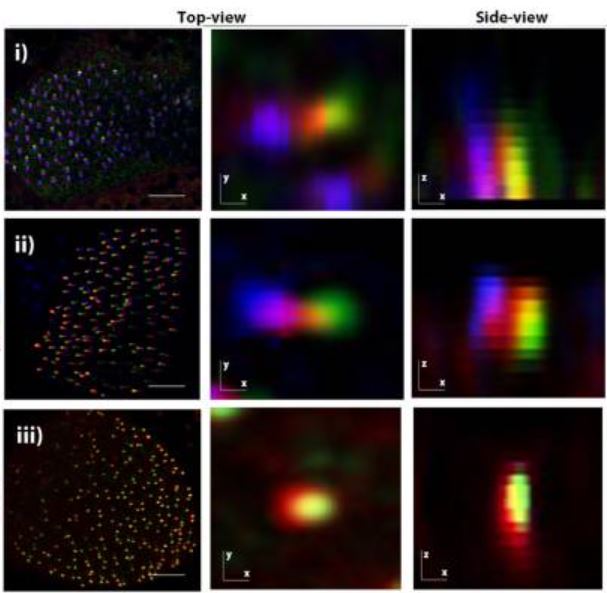
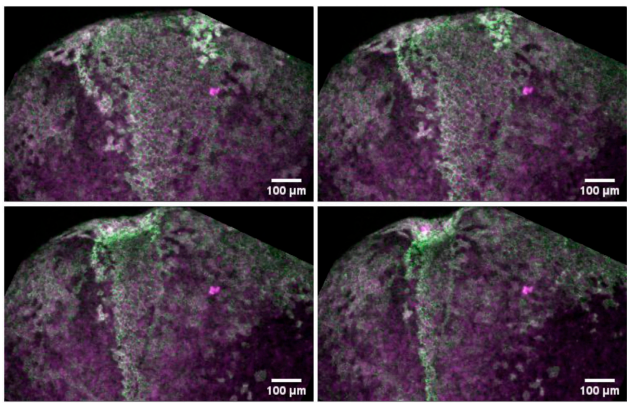
The apical ciliary adhesion complex is established at the basal foot of motile cilia and depends on the microtubule network
2022
Maria Chatzifrangkeskou, Paris A. Skourides
Scientific Reports
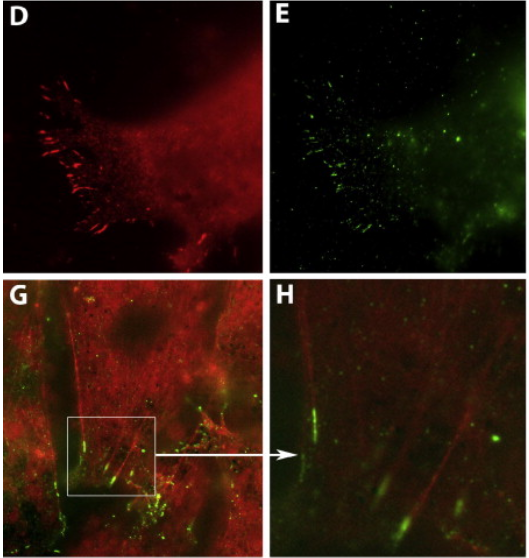
The Ciliary Adhesion (CA) complex forms in close association with the basal bodies of cilia during the early stages of ciliogenesis and is responsible for mediating complex interactions with the actin networks of multiciliated cells (MCCs). However, its precise localization with respect to basal body accessory structures and the interactions that lead to its establishment in MCCs are not well understood. Here, we studied the distribution of the CA proteins using super-resolution imaging and possible interactions with the microtubule network. The results of this study reveal that the apical CA complex forms at the distal end of the basal foot and depends on microtubules. Our data also raise the possibility that CAs may have additional roles in the regulation of the organization of the microtubule network of MCCs.
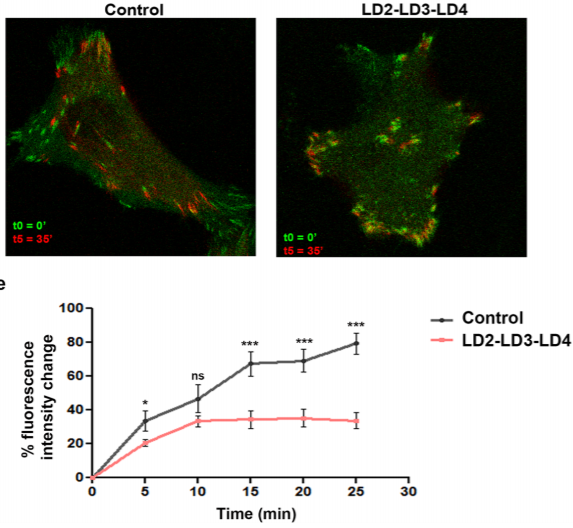
FAK displacement from focal adhesions: a promising strategy to target processes implicated in cancer progression and metastasis
2021
Ioanna Antoniades, Maria Kyriakou, Anna Charalambous, Katerina Kalalidou, Andri Christodoulou, Maria Christoforou, Paris Skourides
Cell Commun Signal.
We took advantage of the well-characterized interactions between the paxillin LD motifs and the FAK FAT domain and generated a polypeptide (LD2-LD3-LD4) expected to compete with interactions with paxillin. Co-immunoprecipitation experiments were performed to examine the interaction between the LD2-LD3-LD4 polypeptide and FAK. The effects of LD2-LD3-LD4 in the localization and functions of FAK, as well as FA composition, were evaluated using quantitative immunofluorescence, cell fractionation, FA isolation and Western Blot analysis. Live cell imaging, as well as 2-D migration and cell invasion assays were used to examine the effects on FA turnover and tumor cell migration and invasion.
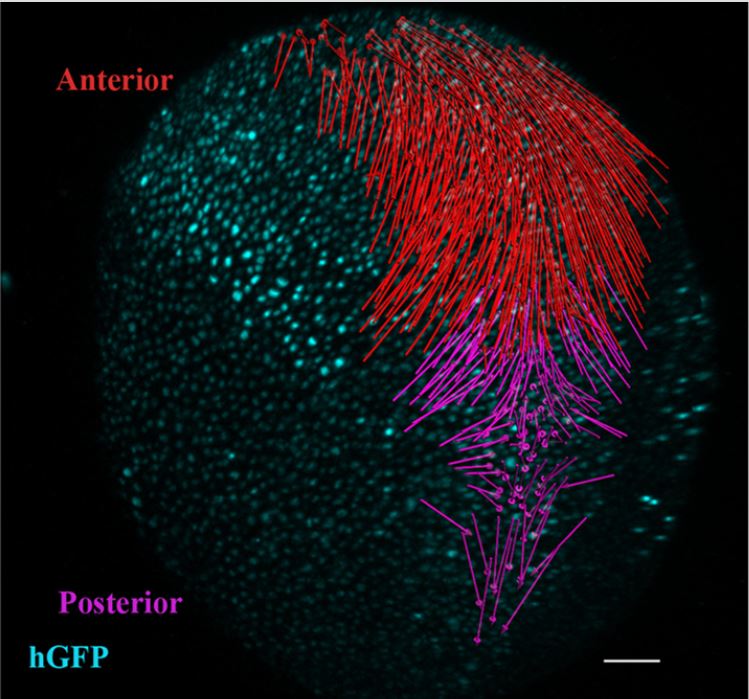
Distinct spatiotemporal contribution of morphogenetic events and mechanical tissue coupling during Xenopus neural tube closure
2022
Neophytos Christodoulou, Paris A. Skourides
Development
Neural tube closure (NTC) is a fundamental process during vertebrate development and is indispensable for the formation of the central nervous system. Here, using Xenopus laevis embryos, live imaging, single-cell tracking, optogenetics and loss of function experiments we examine the roles of convergent extension and apical constriction, and define the role of the surface ectoderm during NTC. We show that NTC is a two-stage process with distinct spatiotemporal contributions of convergent extension and apical constriction at each stage. Convergent extension takes place during the first stage and is spatially restricted at the posterior tissue, while apical constriction occurs during the second stage throughout the neural plate. We go on to show that the surface ectoderm is mechanically coupled with the neural plate and its movement during NTC is driven by neural plate morphogenesis. Last, we show that increase of surface ectoderm resistive forces is detrimental for neural plate morphogenesis.
Adherens junctions stimulate and spatially guide integrin activation and extracellular matrix deposition
July/2022
Rania Hadjisavva, Ouranio Anastasiou, Pantelis S. Ioannou, Maria Zheltkova, Paris A. Skourides
Cadherins and integrins are intrinsically linked through the actin cytoskeleton and are largely responsible for the mechanical integrity and organization of tissues. We show that cadherin clustering stimulates and spatially guides integrin activation. Adherens junction (AJ)-associated integrin activation depends on locally generated tension and does not require extracellular matrix ligands. It leads to the creation of primed integrin clusters, which spatially determine where focal adhesions will form if ligands are present and where ligands will be deposited. AJs that display integrin activation are targeted by microtubules facilitating their disassembly via caveolin-based endocytosis, showing that integrin activation impacts the stability of the core cadherin complex. Thus, the interplay between cadherins and integrins is more intimate than what was once believed and is rooted in the capacity of active integrins to be stabilized via AJ-generated tension. Altogether, our data establish a mechanism of cross-regulation between cadherins and integrins.
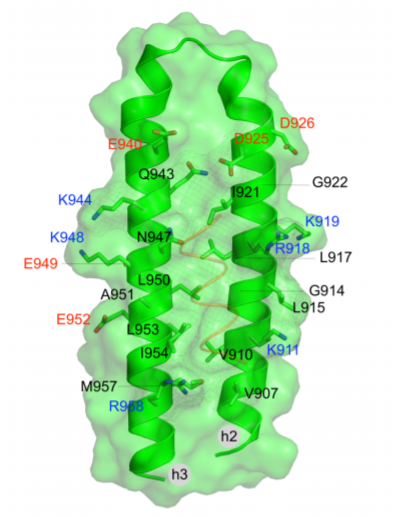
Recognition of LD motifs by the focal adhesion targeting domains of focal adhesion kinase and proline-rich tyrosine kinase 2-beta: Insights from molecular dynamics simulations
2020
Eleni Michael, Savvas Polydorides, Vasilis J Promponas, Paris Skourides, Georgios Archontis
Proteins.
The focal adhesion kinase (FAK) and the proline-rich tyrosine kinase 2-beta (PYK2) are implicated in cancer progression and metastasis and represent promising biomarkers and targets for cancer therapy. FAK and PYK2 are recruited to focal adhesions (FAs) via interactions between their FA targeting (FAT) domains and conserved segments (LD motifs) on the proteins Paxillin, Leupaxin, and Hic-5. A promising new approach for the inhibition of FAK and PYK2 targets interactions of the FAK domains with proteins that promote localization at FAs. Advances toward this goal include the development of surface plasmon resonance, heteronuclear single quantum coherence nuclear magnetic resonance (HSQC-NMR) and fluorescence polarization assays for the identification of fragments or compounds interfering with the FAK-Paxillin interaction. We have recently validated this strategy, showing that Paxillin mimicking polypeptides with 2 to 3 LD motifs displace FAK from FAs and block kinase-dependent and independent functions of FAK, including downstream integrin signaling and FA localization of the protein p130Cas. In the present work we study by all-atom molecular dynamics simulations the recognition of peptides with the Paxillin and Leupaxin LD motifs by the FAK-FAT and PYK2-FAT domains. Our simulations and free-energy analysis interpret experimental data on binding of Paxillin and Leupaxin LD motifs at FAK-FAT and PYK2-FAT binding sites, and assess the roles of consensus LD regions and flanking residues. Our results can assist in the design of effective inhibitory peptides of the FAK-FAT: Paxillin and PYK2-FAT:Leupaxin complexes and the construction of pharmacophore models for the discovery of potential small-molecule inhibitors of the FAK-FAT and PYK2-FAT focal adhesion based functions.
Mitotic cell responses to substrate topological cues are independent of the molecular nature of adhesion
2020
O Anastasiou, R Hadjisavva, P Skourides
Sci Signal
Correct selection of the cell division axis is important for cell differentiation, tissue and organ morphogenesis, and homeostasis. Both integrins, which mediate interactions with extracellular matrix (ECM) components such as fibronectin, and cadherins, which mediate interactions between cells, are implicated in the determination of spindle orientation. We found that both cadherin- and integrin-based adhesion resulted in cell divisions parallel to the attachment plane and elicited identical spindle responses to spatial adhesive cues. This suggests that adhesion topology provides purely mechanical spatial cues that are independent of the molecular nature of the interaction or signaling from adhesion complexes. We also demonstrated that cortical integrin activation was indispensable for correct spindle orientation on both cadherin and fibronectin substrates. These data suggest that spindle orientation responses to adhesion topology are primarily a result of force anisotropy on the cell cortex and show that integrins play a central role in this process that is distinct from their role in cell-ECM interactions.
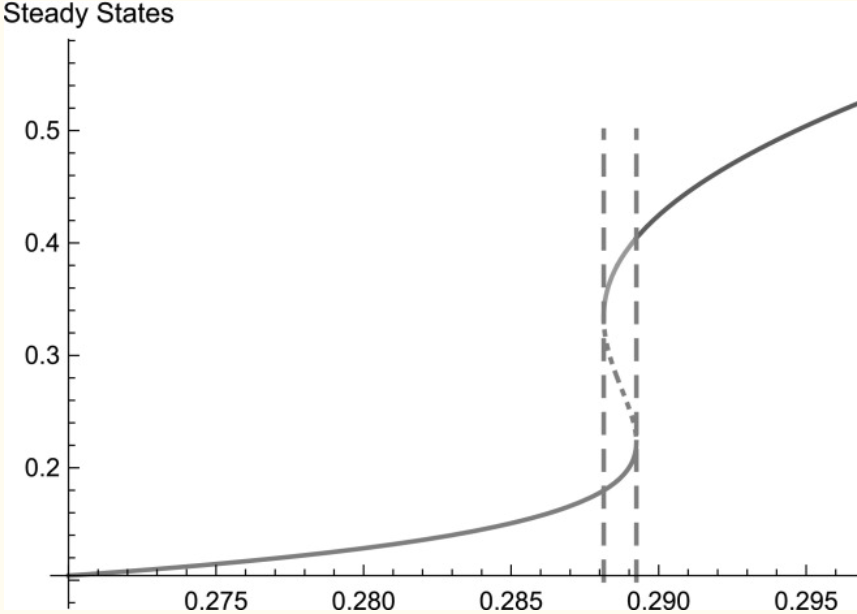
A simple mechanochemical model for calcium signalling in embryonic epithelial cells
2019
K Kaouri, PK Maini, P Skourides, N Christodoulou
J Math Biol.
Calcium signalling is one of the most important mechanisms of information propagation in the body. In embryogenesis the interplay between calcium signalling and mechanical forces is critical to the healthy development of an embryo but poorly understood. Several types of embryonic cells exhibit calcium-induced contractions and many experiments indicate that calcium signals and contractions are coupled via a two-way mechanochemical feedback mechanism. We present a new analysis of experimental data that supports the existence of this coupling during apical constriction. We then propose a simple mechanochemical model, building on early models that couple calcium dynamics to the cell mechanics and we replace the hypothetical bistable calcium release with modern, experimentally validated calcium dynamics. We assume that the cell is a linear, viscoelastic material and we model the calcium-induced contraction stress with a Hill function, i.e. saturating at high calcium levels. We also express, for the first time, the "stretch-activation" calcium flux in the early mechanochemical models as a bottom-up contribution from stretch-sensitive calcium channels on the cell membrane. We reduce the model to three ordinary differential equations and analyse its bifurcation structure semi-analytically as two bifurcation parameters vary-the [Formula: see text] concentration, and the "strength" of stretch activation, [Formula: see text]. The calcium system ([Formula: see text], no mechanics) exhibits relaxation oscillations for a certain range of [Formula: see text] values.
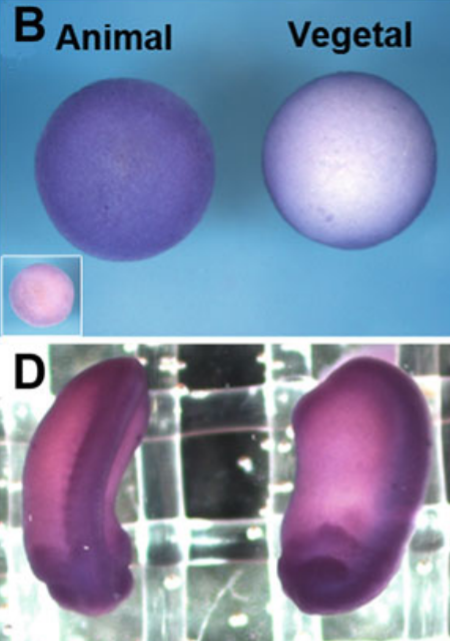
Determining Temporal and Spatial Expression of Calpains in Amphibians
2019
A Charalambous, I Antoniades, N Christodoulou, S Zanardelli, and Skourides PA
Methods Mol Biol.
Calpains are a family of calcium-dependent intracellular cysteine proteases that regulate important physiological processes by substrate cleavage. Despite the fact that Calpains have been identified in the Xenopus genome, their expression patterns and role have not been characterized. Therefore, herein, we describe two methods to determine temporal and spatial expression of Calpain 2 during Xenopus development, namely, RT-PCR and whole-mount in situ hybridization (WISH). In addition, indirect immunofluorescence (IF) is described to determine translocation to the plasma membrane, which correlates with activity levels of Calpain 2.
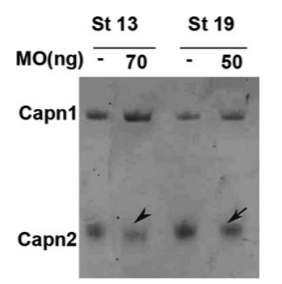
Calpain Inhibition to Determine the Role of Calpains in Embryo Development in Amphibians.
2019
I Antoniades, A Charalambous, N Christodoulou, S Zanardelli, P Skourides
Methods Mol Biol.
Calpains are a family of calcium-dependent intracellular cysteine proteases that regulate important physiological processes by substrate cleavage. Despite the fact that the role of calpains in cell migration and other processes has been extensively studied in vitro, the same does not apply to cell migration and morphogenetic events during embryogenesis, in vivo. Herein, we describe the use of three different methods to selectively block calpain activity in vivo in order to investigate the impact on Xenopus gastrulation and neurulation, namely, a calpain inhibitor, a dominant negative, and a morpholino antisense oligonucleotide (MO). We also provide methods to determine the effectiveness of the calpain inhibition and effect on cell fate specification and morphogenetic movements, during embryogenesis in vivo.
Addressing the Functional Determinants of FAK during Ciliogenesis in Multiciliated Cells
2017
Antoniades I, Stylianou P, Christodoulou N, Skourides PA.
J Biol Chem.
We previously identified focal adhesion kinase (FAK) as an important regulator of ciliogenesis in multiciliated cells. FAK and other focal adhesion (FA) proteins associate with the basal bodies and their striated rootlets and form complexes named ciliary adhesions (CAs). CAs display similarities with FAs but are established in an integrin independent fashion and are responsible for anchoring basal bodies to the actin cytoskeleton during ciliogenesis as well as in mature multiciliated cells. FAK down-regulation leads to aberrant ciliogenesis due to impaired association between the basal bodies and the actin cytoskeleton, suggesting that FAK is an important regulator of the CA complex. However, the mechanism through which FAK functions in the complex is not clear, and in this study we examined the role of this protein in both ciliogenesis and ciliary function. We show that localization of FAK at CAs depends on interactions taking place at the amino-terminal (FERM) and carboxyl-terminal (FAT) domains and that both domains are required for proper ciliogenesis and ciliary function. Furthermore, we show that an interaction with another CA protein, paxillin, is essential for correct localization of FAK in multiciliated cells. This interaction is indispensable for both ciliogenesis and ciliary function. Finally, we provide evidence that despite the fact that FAK is in the active, open conformation at CAs, its kinase activity is dispensable for ciliogenesis and ciliary function revealing that FAK plays a scaffolding role in multiciliated cells. Overall these data show that the role of FAK at CAs displays similarities but also important differences compared with its role at FAs.
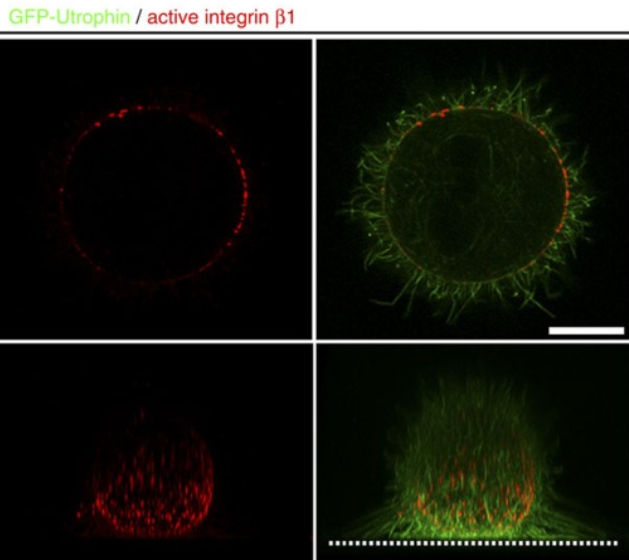
A ligand-independent integrin β1 mechanosensory complex guides spindle orientation.
2016
Petridou NI, Skourides PA.
Nat Commun.
Control of spindle orientation is a fundamental process for embryonic development, morphogenesis and tissue homeostasis, while defects are associated with tumorigenesis and other diseases. Force sensing is one of the mechanisms through which division orientation is determined. Here we show that integrin β1 plays a critical role in this process, becoming activated at the lateral regions of the cell cortex in a ligand-independent manner. This activation is force dependent and polar, correlating with the spindle capture sites. Inhibition of integrin β1 activation on the cortex and disruption of its asymmetric distribution leads to spindle misorientation, even when cell adhesion is β1 independent. Examining downstream targets reveals that a cortical mechanosensory complex forms on active β1, and regulates spindle orientation irrespective of cell context. We propose that ligand-independent integrin β1 activation is a conserved mechanism that allows cell responses to external stimuli.
Cell-Autonomous Ca(2+) Flashes Elicit Pulsed Contractions of an Apical Actin Network to Drive Apical Constriction during Neural Tube Closure.
2015
Christodoulou N, Skourides PA.
Cell Rep.
Neurulation is a critical period in all vertebrates and results in the formation of the neural tube, which gives rise to the CNS. Apical constriction is one of the fundamental morphogenetic movements that drives neural tube closure. Using live imaging, we show that apical constriction during the neurulation is a stepwise process driven by cell-autonomous and asynchronous contraction pulses followed by stabilization steps. Our data suggest that contraction events are triggered by cell-autonomous Ca(2+) flashes and are driven by a transient contractile apical pool of actin. In addition, we provide evidence that the cell autonomy and asynchrony of contraction are required for the correct spatial distribution of constriction and, as a result, are critical for tissue morphogenesis. Finally, we identify Calpain2 as a regulator of apical constriction and show that it is required for the stabilization step, but is dispensable during contraction.
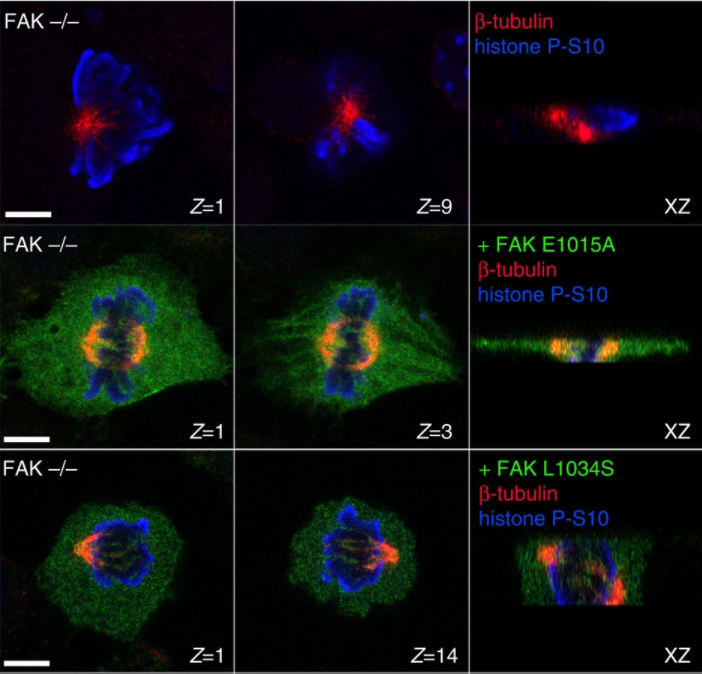
FAK transduces extracellular forces that orient the mitotic spindle and control tissue morphogenesis.
2014
Petridou NI, Skourides PA.
Nat Commun.
Spindle orientation is critical for proper morphogenesis of organs and tissues as well as for the maintenance of tissue morphology. Although significant progress has been made in understanding the mechanisms linking the cell cortex to the spindle and the well-documented role that extracellular forces play in spindle orientation, how such forces are transduced to the cortex remains poorly understood. Here we report that focal adhesion kinase (FAK) is necessary for correct spindle orientation and as a result, indispensable for proper epithelial morphogenesis in the vertebrate embryo. We show that FAK's role in spindle orientation is dependent on its ability to localize at focal adhesions and its interaction with paxillin, but is kinase activity independent. Finally, we present evidence that FAK is required for external force-induced spindle reorientation, suggesting that FAK's involvement in this process stems from a role in the transduction of external forces to the cell cortex.
20210404090409.jpg)
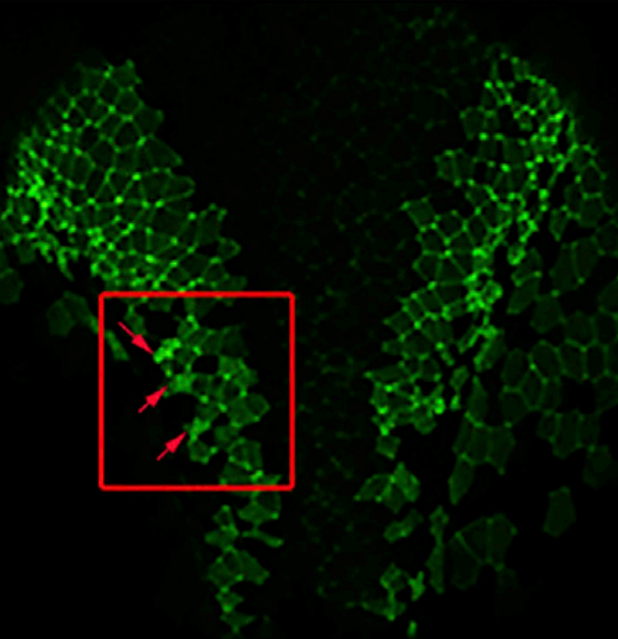
Making the connection: ciliary adhesion complexes anchor Basal bodies to the actin cytoskeleton.
2014
Antoniades I, Stylianou P, Skourides PA.
Dev Cell.
Cilia have been associated with diverse developmental and physiological processes, and defects in cilia underlie a number of genetic conditions. Several lines of evidence support a critical role of the actin cytoskeleton in ciliogenesis and ciliary function. Here, we show that well-characterized focal adhesion (FA) proteins, including FAK, Paxillin, and Vinculin, associate with the basal bodies of multiciliated cells and form complexes (CAs) that interact with the actin cytoskeleton. FAK downregulation leads to ciliogenesis defects similar to those observed when the actin cytoskeleton is disrupted, including defects in basal body migration, docking, and spacing, suggesting that CAs link basal bodies to the actin cytoskeleton. The important role of FA proteins in ciliogenesis leads us to propose that evolutionarily FA proteins, many of which are found in primitive flagellated unicellular eukaryotes, may have originally evolved to perform functions at flagella and were later co-opted for use in cell adhesion.
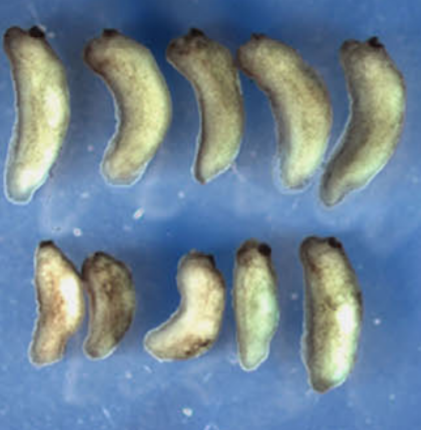
Calpain2 protease: A new member of the Wnt/Ca2+ pathway modulating convergent extension movements in Xenopus.
2013
Zanardelli S, Christodoulou N, Skourides
Dev Biol.
Calpains are a family of calcium-dependent intracellular cysteine proteases that regulate several physiological processes by limited cleavage of different substrates. The role of Calpain2 in embryogenesis is not clear with conflicting evidence from a number of mouse knockouts. Here we report the temporal and spatial expression of Calpain2 in Xenopus laevis embryos and address its role in Xenopus development. We show that Calpain2 is expressed maternally with elevated expression in neural tissues and that Calpain2 activity is spatially and temporally regulated. Using a Calpain inhibitor, a dominant negative and a morpholino oligonoucleotide we demonstrate that impaired Calpain2 activity results in defective convergent extension both in mesodermal and neural tissues. Specifically, Calpain2 downregulation results in loss of tissue polarity and blockage of mediolateral intercalation in Keller explants without affecting adherens junction turnover. We further show that Calpain2 is activated in response to Wnt5a and that the inhibitory effect of Wnt5a expression on animal cap elongation can be rescued by blocking Calpain2 function. This suggests that Calpain2 activity needs to be tightly regulated during convergent extension. Finally we show that expression of Xdd1 blocks the membrane translocation of Calpain2 suggesting that Calpain2 activation is downstream of Dishevelled. Overall our data show that Calpain2 activation through the Wnt/Ca(2+) pathway and Dishevelled can modulate convergent extension movements.
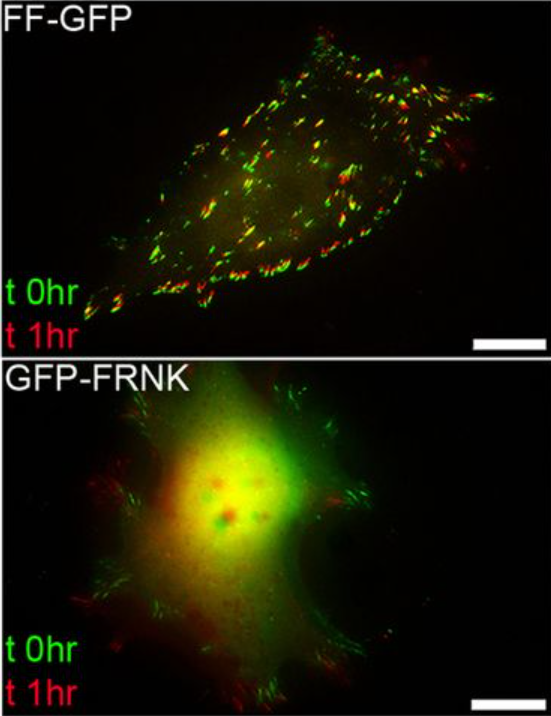
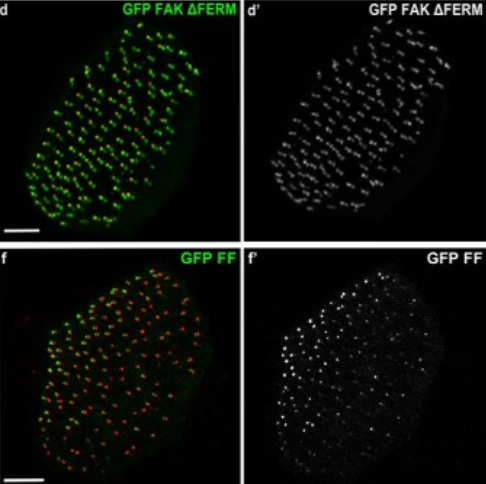
A dominant-negative provides new insights into FAK regulation and function in early embryonic morphogenesis.
2013
Petridou NI, Stylianou P, Skourides PA.
Development
FAK is a non-receptor tyrosine kinase involved in a wide variety of biological processes and crucial for embryonic development. In this manuscript, we report the generation of a new FAK dominant negative (FF), composed of the C terminus (FRNK) and the FERM domain of the protein. FF, unlike FRNK and FERM, mimics the localization of active FAK in the embryo, demonstrating that both domains are necessary to target FAK to its complexes in vivo. We show that the FERM domain has a role in the recruitment of FAK on focal adhesions and controls the dynamics of the protein on these complexes. Expression of FF blocks focal adhesion turnover and, unlike FRNK, acts as a dominant negative in vivo. FF expression in Xenopus results in an overall phenotype remarkably similar to the FAK knockout in mice, including loss of mesodermal tissues. Expression of FF in the animal cap revealed a previously unidentified role of FAK in early morphogenesis and specifically epiboly. We show that a fibronectin-derived signal transduced by FAK governs polarity and cell intercalation. Finally, failure of epiboly results in severe gastrulation problems that can be rescued by either mechanical or pharmacological relief of tension within the animal cap, demonstrating that epiboly is permissive for gastrulation. Overall, this work introduces a powerful new tool for the study of FAK, uncovers new roles for FAK in morphogenesis and reveals new mechanisms through which the FERM domain regulates the localization and dynamics of FAK.
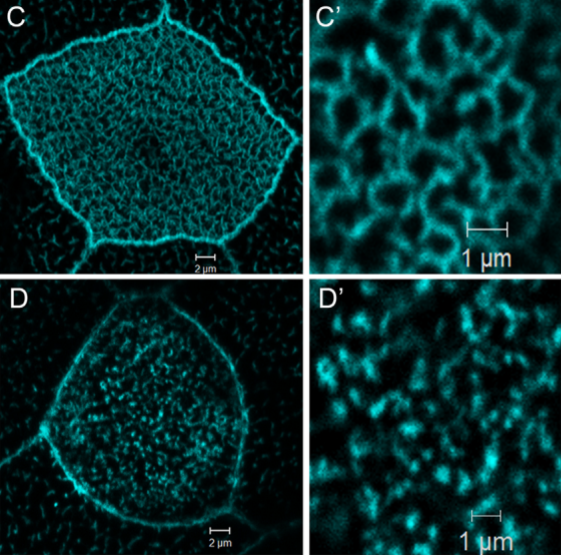
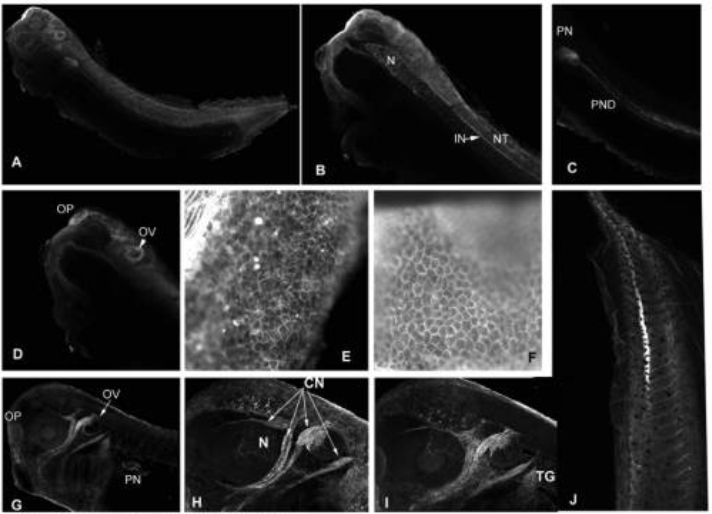
Xenopus laevis nucleotide binding protein 1 (xNubp1) is important for convergent extension movements and controls ciliogenesis via regulation of the actin cytoskeleton.
2013
Ioannou A, Santama N, Skourides PA.
Dev Biol.
Nucleotide binding protein 1 (Nubp1) is a highly conserved phosphate loop (P-loop) ATPase involved in diverse processes including iron-sulfur protein assembly, centrosome duplication and lung development. Here, we report the cloning, expression and functional characterization of Xenopus laevis Nubp1. We show that xNubp1 is expressed maternally, displays elevated expression in neural tissues and is required for convergent extension movements and neural tube closure. In addition, xNubp1knockdown leads to defective ciliogenesis of the multi-ciliated cells of the epidermis as well as the monociliated cells of the gastrocoel roof plate. Specifically, xNubp1 is required for basal body migration, spacing and docking in multi-ciliated cells and basal body positioning and axoneme elongation in monociliated gastrocoel roof plate cells. Live imaging of the different pools of actin and basal body migration during the process of ciliated cell intercalation revealed that two independent pools of actin are present from the onset of cell intercalation; an internal network surrounding the basal bodies, anchoring them to the cell cortex and an apical pool of punctate actin which eventually matures into the characteristic apical actin network. We show that xNubp1 colocalizes with the apical actin network of multiciliated cells and that problems in basal body transport in xNubp1 morphants are associated with defects of the internal network of actin, while spacing and polarity issues are due to a failure of the apical and sub-apical actin pools to mature into a network. Effects of xNubp1 knockdown on the actin cytoskeleton are independent of RhoA localization and activation, suggesting that xNubp1 may have a direct role in the regulation of the actin cytoskeleton.
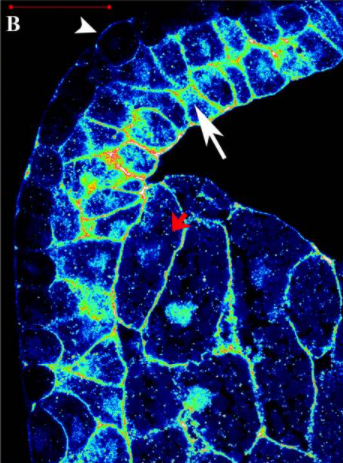
Activation of endogenous FAK via expression of its amino terminal domain in Xenopus embryos.
2012
Petridou NI, Stylianou P, Christodoulou N, Rhoads D, Guan JL, Skourides PA.
PLoS One
The Focal Adhesion Kinase is a well studied tyrosine kinase involved in a wide number of cellular processes including cell adhesion and migration. It has also been shown to play important roles during embryonic development and targeted disruption of the FAK gene in mice results in embryonic lethality by day 8.5. Here we examined the pattern of phosphorylation of FAK during Xenopus development and found that FAK is phosphorylated on all major tyrosine residues examined from early blastula stages well before any morphogenetic movements take place. We go on to show that FRNK fails to act as a dominant negative in the context of the early embryo and that the FERM domain has a major role in determining FAK's localization at the plasma membrane. Finally, we show that autonomous expression of the FERM domain leads to the activation of endogenous FAK in a tyrosine 397 dependent fashion.
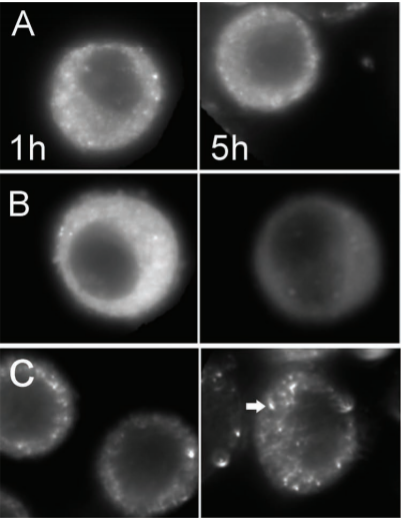
Comparing Intracellular Stability and Targeting of Sulfobetaine Quantum Dots with Other Surface Chemistries in Live Cells.
2012
Muro E, Fragola A, Pons T, Lequeux N, Ioannou A, Skourides P, Dubertret B.
Small
The in vivo labeling of intracellular components with quantum dots (QDs) is very limited because of QD aggregation in the cell cytoplasm and/or QD confinement into lysosomal compartments. In order to improve intracellular targeting with QDs, various surface chemistries and delivery methods have been explored, but they have not yet been compared systematically with respect to the QD intracellular stability. In this work, the intracellular aggregation kinetics of QDs for three different surface chemistries based on ligand exchange or encapsulation with amphiphilic polymers are compared. For each surface chemistry, three delivery methods for bringing the nanoparticles into the cells are compared: electroporation, microinjection, and pinocytosis. It is concluded that the QD intracellular aggregation behavior is strongly dependent on the surface chemistry. QDs coated with dihydrolipoic acid-sulfobetaine (DHLA-SB) ligands diffuse freely in cells for longer periods of time than for QDs in the other chemistries tested, and they can access all cytoplasmic compartments. Even when conjugated to streptavidin, these DHLA-SB QDs remain freely diffusing inside the cytoplasm and unaggregated, and they are able to reach a biotinylated target inside HeLa cells. Such labeling was more efficient when compared to commercial streptavidin-conjugated QDs, which may be due to the smaller size of DHLA-SB QDs and/or to their superior intracellular stability.
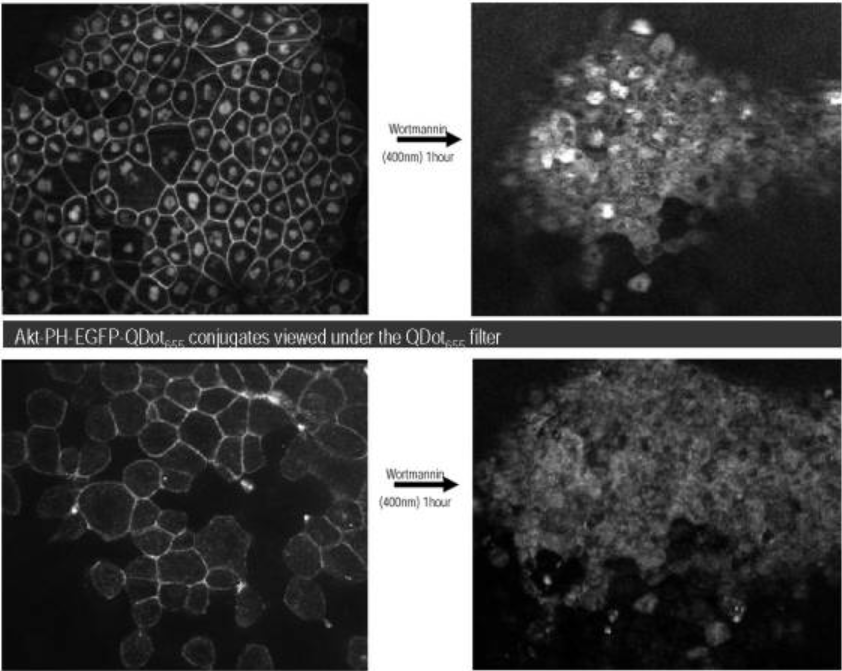
Split-Inteins for Simultaneous, Site-Specific Conjugation of Quantum Dots to Multiple Protein Targets In vivo.
2011
Charalambous A, Antoniades I, Christodoulou N, Skourides PA.
Journal of Nanobiotechnology
Proteins labelled with Quantum Dots (QDs) can be imaged over long periods of time with ultrahigh spatial and temporal resolution, yielding important information on the spatiotemporal dynamics of proteins within live cells or in vivo. However one of the major problems regarding the use of QDs for biological imaging is the difficulty of targeting QDs onto proteins. We have recently developed a DnaE split intein-based method to conjugate Quantum Dots (QDs) to the C-terminus of target proteins in vivo. In this study, we expand this approach to achieve site-specific conjugation of QDs to two or more proteins simultaneously with spectrally distinguishable QDs for multiparameter imaging of cellular functions.
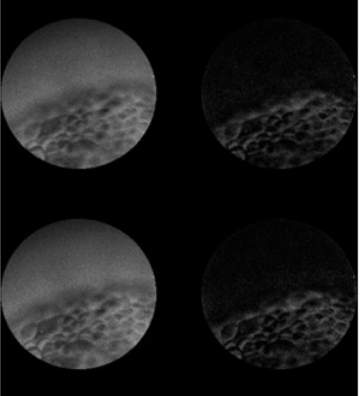
Single-Shot Optical Sectioning using Two-Color Probes in HiLo Fluorescence Microscopy.
2011
Eleonora Muro, Pierre Vermeulen, Andriani Ioannou, Paris Skourides, Benot Dubertret, Alexandra Fragola and Vincent Loriette
Biophysical Journal
We describe a wide-field fluorescence microscope setup which combines HiLo microscopy technique with the use of a two-color fluorescent probe. It allows one-shot fluorescence optical sectioning of thick biological moving sample which is illuminated simultaneously with a flat and a structured pattern at two different wavelengths. Both homogenous and structured fluorescence images are spectrally separated at detection and combined similarly with the HiLo microscopy technique. We present optically sectioned full-field images of Xenopus laevis embryos acquired at 25 images/s frame rate.
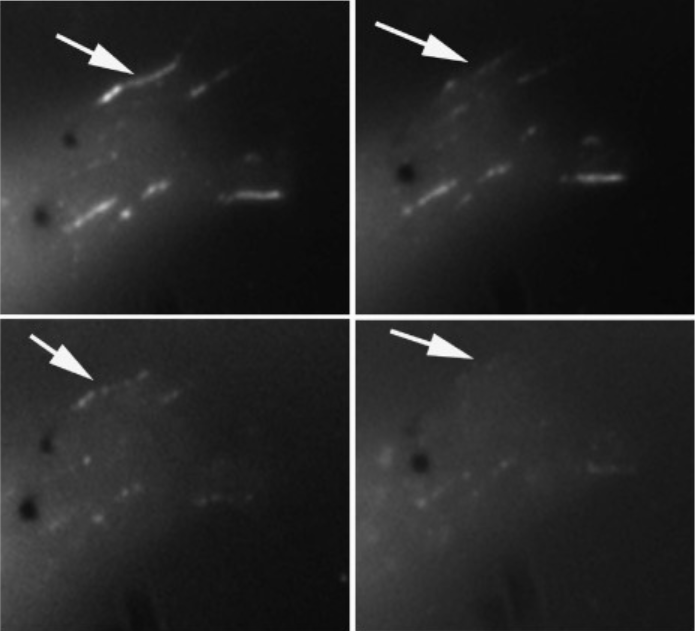
Intein-mediated site-specific conjugation of Quantum Dots to proteins in vivo.
2009
Charalambous A, Andreou M, Skourides PA.
Journal of Nanobiotechnology
We describe an intein based method to site-specifically conjugate Quantum Dots (QDs) to target proteins in vivo. This approach allows the covalent conjugation of any nanostructure and/or nanodevice to any protein and thus the targeting of such material to any intracellular compartment or signalling complex within the cells of the developing embryo. We genetically fused a pleckstrin-homology (PH) domain with the N-terminus half of a split intein (IN). The C-terminus half (IC) of the intein was conjugated to QDs in vitro. IC-QD's and RNA encoding PH-IN were microinjected into Xenopus embryos. In vivo intein-splicing resulted in fully functional QD-PH conjugates that could be monitored in real time within live embryos. Use of Near Infra Red (NIR)-emitting QDs allowed monitoring of QD-conjugates within the embryo at depths where EGFP is undetectable demonstrating the advantages of QD's for this type of experiment. In conclusion, we have developed a novel in vivo methodology for the site-specific conjugation of QD's and other artificial structures to target proteins in different intracellular compartments and signaling complexes.
Imaging morphogenesis, in Xenopus with Quantum Dot nanocrystals.
2009
Stylianou P, Skourides PA.
Mech Dev.
Mesoderm migration is a well studied morphogenetic movement that takes place during Xenopus gastrulation. The study of mesoderm migration and other morphogenetic movements has been primarily based on in vitro assays due to the inability to image deep tissue movements in the opaque embryo. We are the first to report the use of Near Infra Red Quantum Dots (NIR QD's) to image mesoderm migration in vivo with single cell resolution and provide quantitative in vivo data regarding migration rates. In addition we use QD's to address the function of the focal adhesion kinase (FAK) in this movement. Inhibition of FAK blocks mesoderm spreading and migration both in vitro and in vivo without affecting convergent extension highlighting the molecular differences between the two movements. These results provide new insights about the role of FAK and of focal adhesions during gastrulation and provide a new tool for the study of morphogenesis in vivo.
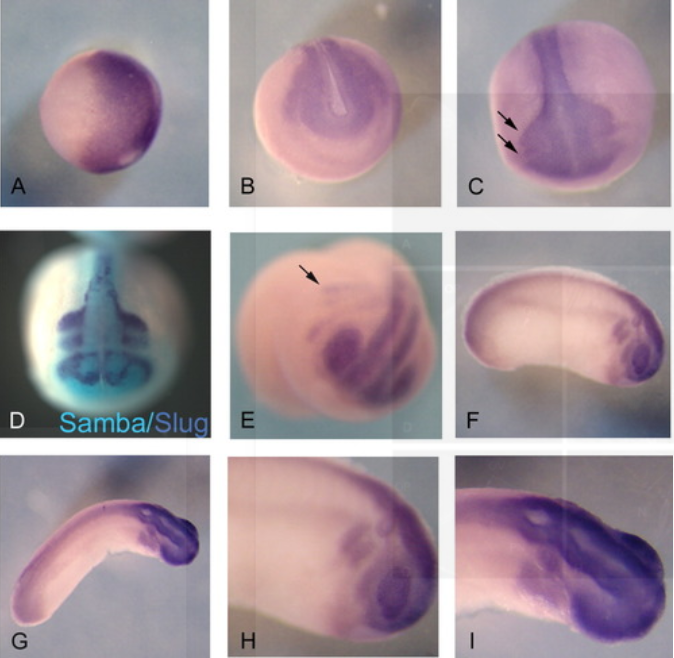
Samba, a Xenopus hnRNP expressed in neural and neural crest tissues.
2009
Yan CY, Skourides P, Chang C, Brivanlou A.
Developmental Dynamics
RNA binding proteins regulate gene expression at the posttranscriptional level and play important roles in embryonic development. Here, we report the cloning and expression of Samba, a Xenopus hnRNP that is maternally expressed and persists at least until tail bud stages. During gastrula stages, Samba is enriched in the dorsal regions. Subsequently, its expression is elevated only in neural and neural crest tissues. In the latter, Samba expression overlaps with that of Slug in migratory neural crest cells. Thereafter, Samba is maintained in the neural crest derivatives, as well as other neural tissues, including the anterior and posterior neural tube and the eyes. Overexpression of Samba in the animal pole leads to defects in neural crest migration and cranial cartilage development. Thus, Samba encodes a Xenopus hnRNP that is expressed early in neural and neural crest derivatives and may regulate crest cells migratory behavior.
Spatially and temporally regulated alpha6 integrin cleavage during Xenopus laevis development.
2007
Demetriou MC, Stylianou P, Andreou M, Yiannikouri O, Tsaprailis G, Cress AE, Skourides P.
Biochem Biophys Res Commun.
The alpha6 integrin is essential for early nervous system development in Xenopus laevis. We have previously reported a uPA cleaved form of integrin alpha6 (alpha6p), in invasive human prostate cancer tissue, whose presence correlates with increased migration and invasive capacity. We now report that alpha6 is cleaved during the normal development of Xenopus in a spatially and temporally controlled manner. In addition, unlike normal mammalian tissues, which lack alpha6p, the major form of the alpha6 integrin present in adult Xenopus is alpha6p. The protease responsible for the cleavage in mammals, uPA, is not involved in the cleavage of Xenopus alpha6. Finally, overexpression of a mammalian alpha6 mutant which cannot be cleaved leads to developmental abnormalities suggesting a potential role for the cleavage in development.
In vivo imaging of quantum dots encapsulated in phospholipid micelles
2002
Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A.
Science
Fluorescent semiconductor nanocrystals (quantum dots) have the potential to revolutionize biological imaging, but their use has been limited by difficulties in obtaining nanocrystals that are biocompatible. To address this problem, we encapsulated individual nanocrystals in phospholipid block-copolymer micelles and demonstrated both in vitro and in vivo imaging. When conjugated to DNA, the nanocrystal-micelles acted as in vitro fluorescent probes to hybridize to specific complementary sequences. Moreover, when injected into Xenopus embryos, the nanocrystal-micelles were stable, nontoxic (<5 x 10(9) nanocrystals per cell), cell autonomous, and slow to photobleach. Nanocrystal fluorescence could be followed to the tadpole stage, allowing lineage-tracing experiments in embryogenesis.
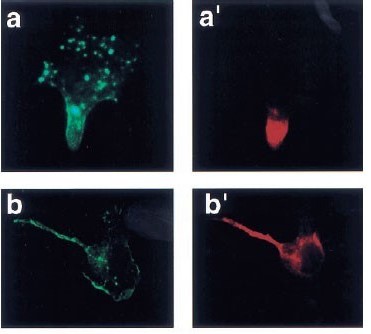
Polarized distribution of Bcr-Abl in migrating myeloid cells and co-localization of Bcr-Abl and its target proteins.
1999
Paris A.Skourides, Samanthi A.Perera, Ruibao Ren
Oncogene
Bcr-Abl plays a critical role in the pathogenesis of Philadelphia chromosome-positive leukemia. Although a large number of substrates and interacting proteins of Bcr-Abl have been identified, it remains unclear whether Bcr-Abl assembles multi-protein complexes and if it does where these complexes are within cells. We have investigated the localization of Bcr-Abl in 32D myeloid cells attached to the extracellular matrix. We have found that Bcr-Abl displays a polarized distribution, colocalizing with a subset of filamentous actin at trailing portions of migrating 32D cells, and localizes on the cortical F-actin and on vesicle-like structures in resting 32D cells. Deletion of the actin binding domain of Bcr-Abl (Bcr-AbI-AD) dramatically enhances the localization of Bcr-Abl on the vesicle-like structures. These distinct localization patterns of Bcr-Abl and Bcr-Abl-AD enabled us to examine the localization of Bcr-Abl substrate and interacting proteins in relation to Bcr-Abl. We found that a subset of biochemically defined target proteins of Bcr-Abl redistributed and co-localized with Bcr-Abl on F-actin and on vesicle-like structures. The co-localization of signaling proteins with Bcr-Abl at its sites of localization supports the idea that Bcr-Abl forms a multi-protein signaling complex, while the polarized distribution and vesicle-like localization of Bcr-Abl may play a role in leukemogenesis.
